Andrew Swallow
Glyphosate is almost certainly the most widely used herbicide in New Zealand but its future is under threat so be smart about how you use it, growers gathered at the Foundation of Arable Research’s main field day this summer were told.
While the message on the day was directed at mostly cropping farmers and their support industry, it is equally applicable to livestock farmers, speakers Phil Rolston and Matilda Gunnarsson of FAR later confirmed to Country-Wide.
“Maintaining both glyphosate efficacy, and the social licence to use it, relies on appropriate and responsible use,” they stressed.
Weather, water and target type/condition all affect performance so should be taken into account when making applications: spraying in low humidity reduces plant uptake as droplets dry too quickly; daylight is needed to help the plant translocate the glyphosate throughout shoots and roots; moderate temperature (15-25C) is best as too hot or cold limits absorption and translocation.
‘Maintaining both glyphosate efficacy, and the social licence to use it, relies on appropriate and responsible use.’
Water needs to be clean and ideally mildly acidic.
“You don’t want it high in magnesium or calcium,” noted Gunnarsson.
Glyphosate formulations, when added to the tank, lower pH so water that is around pH 6 naturally will drop into the recommended pH range for a glyphosate spray solution of 4.5 to 5.0. For example, water used for sprays at the field day site, (Chertsey, Canterbury) is pH 6.1 but with glyphosate added that drops to about 4.8, she said. If pH was likely to be too high, use of an acidifier such as citric or folic acid should be considered.
Clean water is important because clay or organic particles in the spray tank bind to glyphosate molecules rendering them ineffective as a herbicide. Problems often occur where sprayers are filled from rivers or ponds, she noted.
Glyphosate won’t work well on stressed plants due to reduced translocation and it is more effective on young, actively growing plants. When spraying out pasture, be it after grazing or harvesting for seed, “the amount of biomass when you spray is absolutely critical,” Gunnarsson stressed.
Spray too soon after defoliation and an initial kill will be achieved but the herbicide won’t translocate to side shoots/tillers and regrowth will occur, she explained. Overall, the principle is to understand the biology of the target. For example, rhizomatous and deeply rooting weeds such as couch or Californian thistle won’t be controlled well by glyphosate in early spring as there is little translocation from foliage to roots, even if there is enough foliage to target.
While ryegrass resistant to glyphosate has been confirmed in several locations in NZ now, and cases are widespread elsewhere in the world, especially Australia, often failures to work as expected here are found to be down to environmental or application factors.
“Last autumn in South Canterbury there were reports of glyphosate not doing its job in ryegrass after seed crops,” Rolston recalled at the Chertsey field day.
“So we took samples of the products and plants and did a trial at Lincoln using a logarithmic sprayer and they all worked perfectly well, so it can be all about the environment at the time of application. Were the plants under water stress? Was it temperature?”
Samples of apparently glyphosate-resistant ryegrass from nine farms in Selwyn District* had also been found to die when regrown from tillers of the surviving plant and sprayed again, suggesting high temperature at time of application might have been behind the parent plant’s survival.
Rolston also noted there are reports of users applying 2.0 litres/ha regardless of the brand of glyphosate or target, despite formulations containing anything from the old standard 360g/litre of active ingredient, right up to 660g/litre, with a similarly wide range in recommended rates, nearly all of which are over 2.0 litres/ha.
In a trial at Lincoln comparing 2L/ha applications with recommended rates percentage brownout at 35 days was lower at the 2L/ha rate, but only by four percentage points (see table). [NB: not the same as 4%].
One thing all labels have in common is recommending a surfactant to aid product uptake, but again there’s a wide range of what and how much is used on farm, noted Rolston. In the Lincoln trial, where Pulse penetrant was the surfactant used, or not, it significantly improved mean brownout % at 2L/ha, but not at label rates.
The trial is being repeated this spring and summer at various times to try to establish what is driving differences in efficacy reported.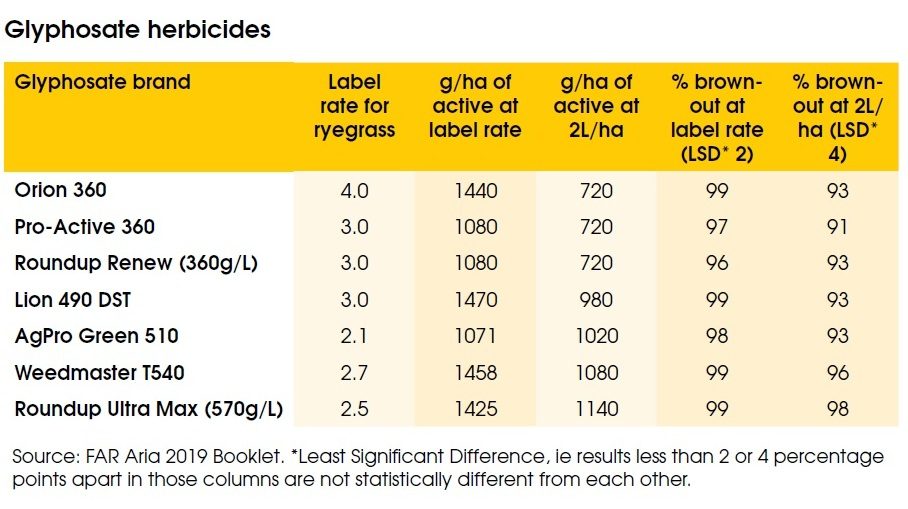
Hazard and risk misunderstood
 Phil Rolston (see main story) reckoned he’d “drawn the short straw” in having to present the glyphosate session. The herbicide is “an incredibly important tool” and the growing public debate about glyphosate use made him nervous it is under increasing threat.
Phil Rolston (see main story) reckoned he’d “drawn the short straw” in having to present the glyphosate session. The herbicide is “an incredibly important tool” and the growing public debate about glyphosate use made him nervous it is under increasing threat.
“We need to use it wisely. That’s really, really important, because public perception is everything, whether it’s right or wrong,” he warned.
Public fears are easily fanned because few people understand the difference between hazard and risk, he added.
Glyphosate, alcohol, and coffee are all hazardous substances. Whether or not they are a risk to health depends on exposure and, for the general public, the risk of exposure to glyphosate is very, very low, he explained.
Adding to the problem in New Zealand is that when registered, the Maximum Residue Limit for glyphosate in cereals was set at the default level, which is “incredibly low”, Rolston said. It is probably much lower than the figure that would have been given had a product-specific MRL for glyphosate been sought. As a consequence, even when used to the letter of the label for pre-harvest weed control, NZ’s MRL can exceeded, alarming the public.
“The UK has an MRL for glyphosate which is at least 10 times higher than here.”
FAR’s newly released guidelines for glyphosate management have a question-mark against use for near-harvest weed control – “check your crop contract” it states – and a cross for pre-harvest dessication.




